Is baf2 an acid or base – When it comes to chemical compounds, understanding their acid-base properties is crucial. In this exploration, we delve into the intriguing case of BaF2, commonly known as barium fluoride, to uncover whether it falls under the acidic or basic category. Join us as we unravel the fascinating world of chemical properties, shedding light on the nature of BaF2 and its behavior in various contexts.
Barium fluoride (BaF2) is an inorganic compound composed of barium (Ba) and fluorine (F) atoms. It is a white, crystalline solid with a high melting point and low solubility in water. BaF2 finds applications in various industries, including glass manufacturing, optics, and metallurgy.
Chemical Properties of BaF2
Barium fluoride (BaF2) is an inorganic compound with a chemical formula that comprises one barium cation (Ba 2+) and two fluoride anions (F –). The molecular structure of BaF2 adopts a cubic crystal lattice, where each barium ion is surrounded by eight fluoride ions, and vice versa.
This arrangement results in a strong ionic bond between the barium and fluorine atoms, giving BaF2 its characteristic properties.
Physical and Chemical Properties
The physical and chemical properties of BaF2 are summarized in the following table:
| Property | Value |
|---|---|
| Formula | BaF2 |
| Molecular Weight | 175.3 g/mol |
| Appearance | White solid |
| Melting Point | 1280 °C |
| Boiling Point | 2260 °C |
| Solubility in Water | 0.16 g/100 mL |
| Crystal Structure | Cubic |
| Band Gap | 11.7 eV |
Acid-Base Properties of BaF2
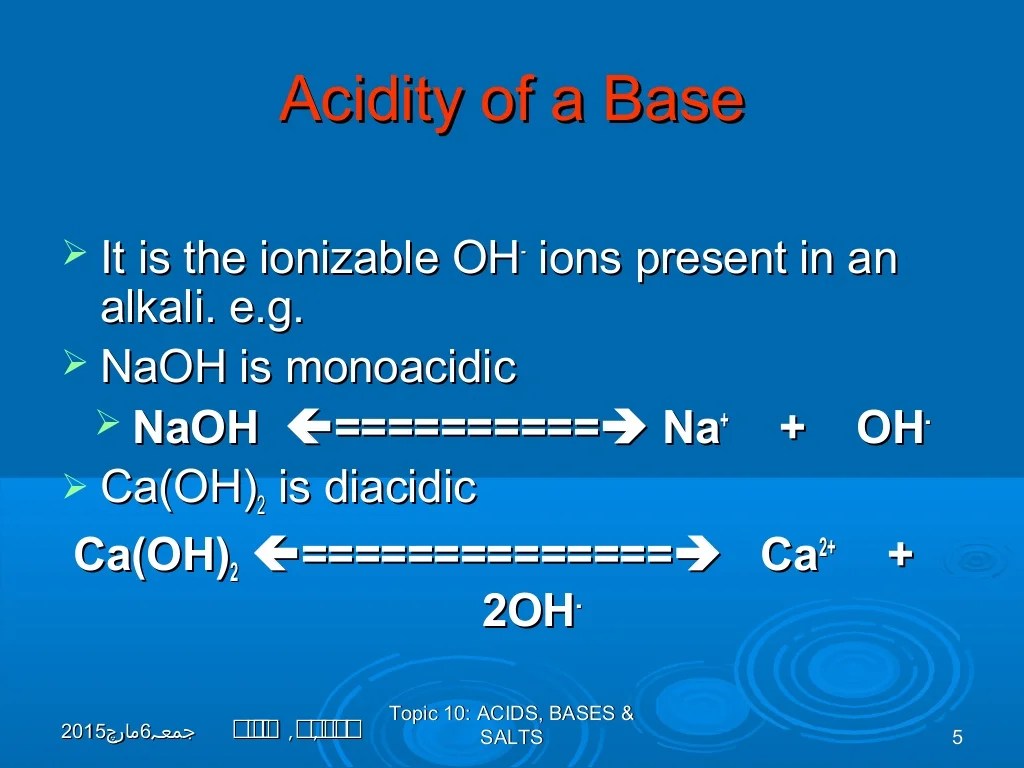
Acids and bases are two fundamental concepts in chemistry that describe the ability of substances to donate or accept protons (H+ ions). According to the Brønsted-Lowry theory, an acid is a substance that can donate a proton, while a base is a substance that can accept a proton.BaF2
is an ionic compound that does not contain any acidic or basic groups. It is formed by the combination of barium ions (Ba2+) and fluoride ions (F-). In water, BaF2 does not undergo any proton transfer reactions, meaning it does not donate or accept protons.
Is BaF2 an acid or a base? That’s a question that can be answered by taking the Omega Psi Phi MSP test . This test is designed to measure your knowledge of basic chemistry, and it can be a helpful way to determine your understanding of the subject.
So, if you’re wondering whether BaF2 is an acid or a base, take the test and find out!
Therefore, BaF2 is considered a neutral salt, which means it is neither acidic nor basic.
Solubility and Hydrolysis of BaF2: Is Baf2 An Acid Or Base
BaF2 exhibits limited solubility in water, dissolving to a small extent to form a saturated solution. The solubility of BaF2 in water is influenced by temperature, with solubility increasing as temperature rises.
Hydrolysis is a chemical process that involves the reaction of a substance with water, leading to the formation of a base and an acid. In the case of BaF2, hydrolysis occurs to a very limited extent, resulting in the formation of barium hydroxide [Ba(OH)2] and hydrofluoric acid [HF].
Extent of BaF2 Hydrolysis and its Implications
The extent of BaF2 hydrolysis is relatively small, with only a small fraction of the dissolved BaF2 molecules undergoing hydrolysis. This limited hydrolysis has several implications:
- Weak Base Formation:The hydrolysis of BaF2 produces a weak base, Ba(OH)2. This means that the solution formed upon dissolving BaF2 in water exhibits a slightly basic pH.
- Buffering Capacity:The presence of both BaF2 and its hydrolysis products, Ba(OH)2 and HF, creates a buffer system in solution. This buffer system helps to resist changes in pH, maintaining a relatively stable pH range.
The limited hydrolysis of BaF2 and the resulting formation of a weak base and buffer system are important factors to consider when using BaF2 in various applications, such as in the production of optical materials or as a flux in metallurgy.
Applications of BaF2
Barium fluoride (BaF2) finds diverse applications in various industries and scientific fields.
Industrial Uses
BaF2 is widely used in the glass and ceramics industry as a fluxing agent. It lowers the melting point of glass mixtures, improving their fluidity and workability. Additionally, BaF2 is employed as an opacifier in the production of enamels and glazes, imparting an opaque white appearance.
Optical Applications, Is baf2 an acid or base
BaF2 possesses exceptional optical properties, making it valuable in optical applications. Its high refractive index and low dispersion render it suitable for use in lenses, prisms, and other optical components. Moreover, BaF2 exhibits excellent transmission in the ultraviolet and infrared regions of the electromagnetic spectrum, making it ideal for applications in spectroscopy and imaging.
Biomedical Research
BaF2 has shown promising potential in biomedical research. Its ability to emit fluorescence when exposed to X-rays has led to its use in medical imaging techniques, such as X-ray computed tomography (CT) and fluoroscopy. Additionally, BaF2 nanoparticles are being explored for targeted drug delivery and as contrast agents in magnetic resonance imaging (MRI).
FAQ
Is BaF2 soluble in water?
BaF2 has low solubility in water.
What is the chemical formula of barium fluoride?
The chemical formula of barium fluoride is BaF2.
What are the industrial applications of BaF2?
BaF2 is used in glass manufacturing, optics, and metallurgy.