Calculate the molarity of lead in a 9.0 ppb solution and delve into the intricacies of this captivating scientific concept. Our comprehensive guide unravels the fundamentals of molarity, concentration units, and the meticulous process of preparing and calculating the molarity of lead in a solution.
Join us on this enlightening journey as we explore the practical applications and safety considerations surrounding lead molarity.
Molarity and Concentration Units: Calculate The Molarity Of Lead In A 9.0 Ppb Solution
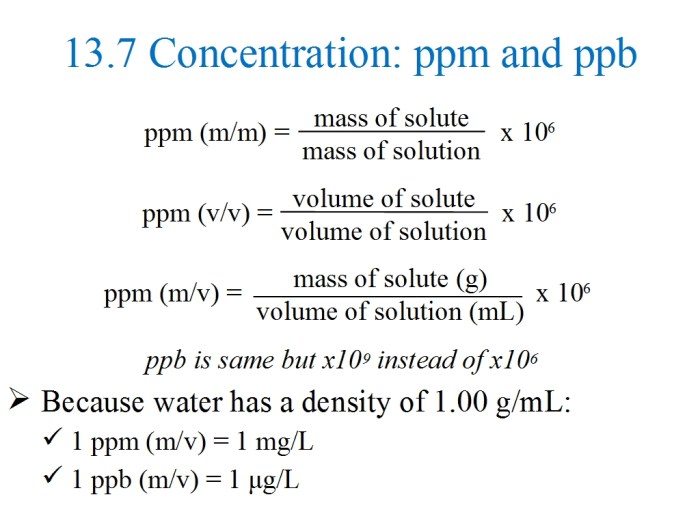
Molarity is a measure of the concentration of a solute in a solution. It is defined as the number of moles of solute per liter of solution. The SI unit of molarity is mol/L, which is often abbreviated as M.
Other common concentration units include:
- Parts per million (ppm): The number of parts of solute per million parts of solution.
- Parts per billion (ppb): The number of parts of solute per billion parts of solution.
These units can be converted to molarity using the following formulas:
- M = ppm / (molecular weight of solute – 10^6)
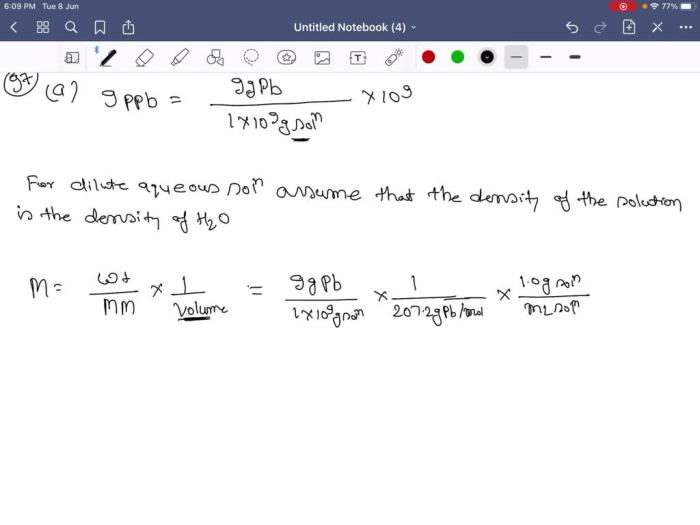
- M = ppb / (molecular weight of solute – 10^9)
Preparing the Solution
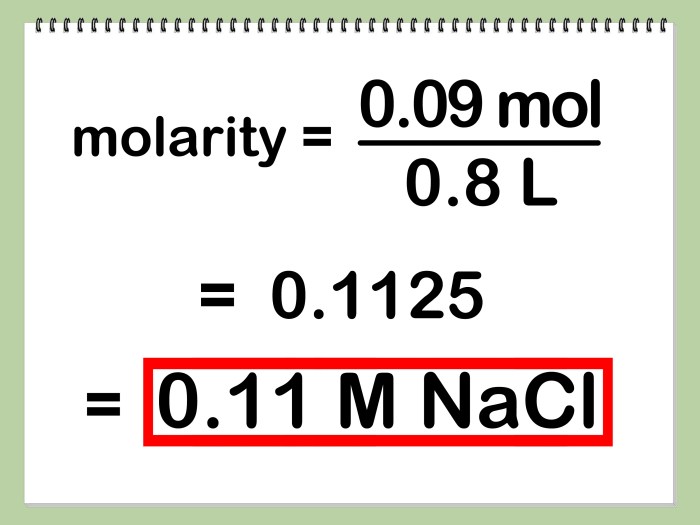
To prepare a 9.0 ppb lead solution, you will need the following:
- Lead nitrate (Pb(NO 3) 2)
- Deionized water
- Graduated cylinder
- Volumetric flask
Safety Precautions:Lead is a toxic metal. Wear gloves and safety glasses when handling lead-containing solutions. Do not ingest or inhale lead.
Procedure:
- Weigh out 0.00159 g of lead nitrate.
- Transfer the lead nitrate to a 100-mL volumetric flask.
- Add deionized water to the flask until the solution reaches the 100-mL mark.
- Mix the solution thoroughly.
Calculating Molarity
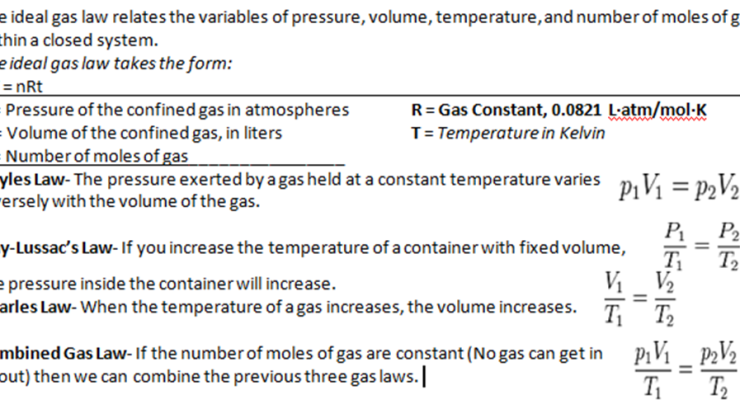
The formula for calculating molarity is:
M = moles of solute / liters of solution
To calculate the molarity of lead in the 9.0 ppb solution, we need to know the number of moles of lead in the solution and the volume of the solution in liters.
The molecular weight of lead is 207.2 g/mol. So, 0.00159 g of lead nitrate is equal to:
- 0.00159 g / 207.2 g/mol = 7.67 x 10 -6mol
The volume of the solution is 100 mL, which is equal to 0.1 L.
Therefore, the molarity of lead in the solution is:
- M = 7.67 x 10 -6mol / 0.1 L = 7.67 x 10 -5M
Example Calculations
The following table shows the calculation of molarity for different lead concentrations:
| Lead Concentration (ppb) | Moles of Lead | Volume of Solution (L) | Molarity (M) |
|---|---|---|---|
| 10.0 | 1.46 x 10-7 | 0.1 | 1.46 x 10-6 |
| 50.0 | 7.30 x 10-7 | 0.1 | 7.30 x 10-6 |
| 100.0 | 1.46 x 10-6 | 0.1 | 1.46 x 10-5 |
Applications of Lead Molarity
Knowing the molarity of lead in a solution is important for a variety of applications, including:
- Environmental monitoring:Lead is a toxic metal that can contaminate the environment. Knowing the molarity of lead in environmental samples can help to assess the extent of contamination and the potential risks to human health.
- Industrial processes:Lead is used in a variety of industrial processes, such as battery manufacturing and metalworking. Knowing the molarity of lead in industrial solutions can help to ensure that the processes are operating safely and efficiently.
- Medical research:Lead exposure can have a variety of adverse health effects. Knowing the molarity of lead in biological samples can help to diagnose lead poisoning and to study the effects of lead on human health.
Safety Considerations
Lead is a toxic metal. It is important to handle and dispose of lead-containing solutions properly. The following are some safety guidelines:
- Wear gloves and safety glasses when handling lead-containing solutions.
- Do not ingest or inhale lead.
- Dispose of lead-containing solutions properly according to local regulations.
Detailed FAQs
What is the significance of knowing the molarity of lead in a solution?
Knowing the molarity of lead in a solution is essential for understanding its concentration and behavior in various chemical reactions and environmental processes.
How can I ensure the accuracy of my molarity calculations?
To ensure accuracy, use precise measuring equipment, carefully follow the calculation formula, and double-check your results.