Dive into the captivating world of AP chemistry gas laws frq, where the behavior of gases unravels before your eyes. Get ready for an exciting journey filled with essential concepts, problem-solving techniques, and real-world applications.
In this comprehensive guide, we’ll explore the fundamental principles governing gases, from the ideal gas law to the kinetic molecular theory. We’ll delve into gas stoichiometry and unravel the mysteries of gas laws, empowering you to conquer the challenges of AP chemistry.
Ideal Gas Law

The ideal gas law is a mathematical equation that describes the behavior of gases under various conditions. It relates the pressure, volume, temperature, and number of moles of a gas sample.
The ideal gas law equation is: PV = nRT where: – P is the pressure of the gas in pascals (Pa) – V is the volume of the gas in cubic meters (m3) – n is the number of moles of gas in moles (mol) – R is the ideal gas constant, which is 8.314 J/mol·K – T is the temperature of the gas in kelvins (K)
The ideal gas law can be used to solve a variety of problems, such as:
- Calculating the pressure of a gas sample when the volume, temperature, and number of moles are known
- Calculating the volume of a gas sample when the pressure, temperature, and number of moles are known
- Calculating the temperature of a gas sample when the pressure, volume, and number of moles are known
- Calculating the number of moles of gas in a sample when the pressure, volume, and temperature are known
The ideal gas law is a useful tool for understanding the behavior of gases. However, it is important to note that the ideal gas law is only an approximation of the real behavior of gases. Real gases deviate from ideal behavior at high pressures and low temperatures.
When it comes to acing the AP Chemistry gas laws FRQ, understanding the fundamentals is crucial. One helpful resource for exam preparation is h5521 470 04 – local ppo . This website provides in-depth explanations and practice problems that align with the exam’s format.
By incorporating this resource into your study plan, you’ll be well-equipped to tackle the gas laws FRQ with confidence.
Kinetic Molecular Theory
The kinetic molecular theory (KMT) is a model that describes the behavior of gases. It is based on the following postulates:* Gases are composed of tiny, spherical particles called molecules.
- The molecules are in constant, random motion.
- The molecules collide with each other and with the walls of their container.
- The average kinetic energy of the molecules is proportional to the absolute temperature of the gas.
The KMT can be used to explain a wide variety of gas properties, including pressure, volume, temperature, and behavior.
Applications of the Kinetic Molecular Theory
The KMT can be used to solve a variety of problems, including:* Predicting the pressure of a gas in a container.
- Calculating the volume of a gas at a given temperature and pressure.
- Determining the temperature of a gas from its pressure and volume.
- Explaining the behavior of gases in chemical reactions.
The KMT is a powerful tool that can be used to understand the behavior of gases. It is a fundamental theory in chemistry and is used in a wide variety of applications.
Gas Stoichiometry
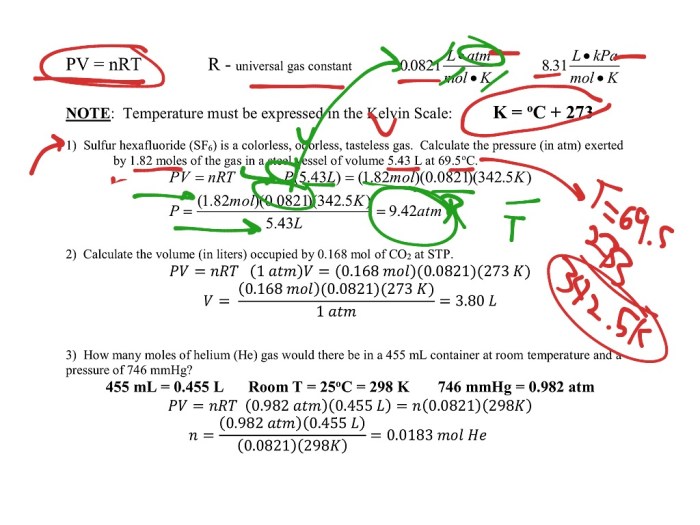
Gas stoichiometry involves using the mole concept to determine the quantitative relationships between gases involved in chemical reactions. It enables us to calculate the mass, volume, or moles of gases based on stoichiometric ratios.
Calculating Mass, Volume, or Moles of Gases, Ap chemistry gas laws frq
To calculate the mass, volume, or moles of gases involved in a reaction, we follow these steps:
- Balance the chemical equation to determine the mole ratios between the reactants and products.
- Use the mole ratio to convert the given quantity (mass, volume, or moles) of one substance to the desired quantity of another substance.
- Apply the ideal gas law or other gas laws to convert between mass, volume, and moles if necessary.
Applications of Gas Stoichiometry
Gas stoichiometry has numerous applications in chemistry, including:
- Determining the limiting reactant in a reaction.
- Predicting the products and their quantities in a chemical reaction.
- Calculating the molar mass of a gas.
- Analyzing the composition of gas mixtures.
- Designing and optimizing industrial processes involving gases.
Gas Laws and Phase Diagrams: Ap Chemistry Gas Laws Frq
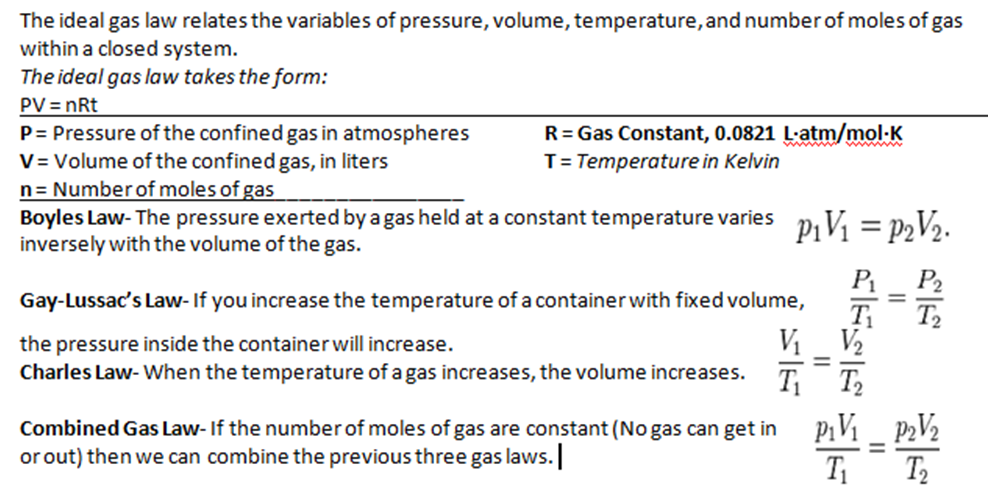
Gas laws describe the relationship between various properties of gases, such as pressure, volume, temperature, and the number of moles. These laws provide a fundamental understanding of the behavior of gases and allow us to predict their properties under different conditions.
Boyle’s Law
Boyle’s law states that the pressure of a gas is inversely proportional to its volume at constant temperature. This means that as the volume of a gas decreases, its pressure increases, and vice versa. Mathematically, Boyle’s law can be expressed as:
P1V 1= P 2V 2
Where P 1and V 1represent the initial pressure and volume, and P 2and V 2represent the final pressure and volume, respectively.
Charles’s Law
Charles’s law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure. This means that as the temperature of a gas increases, its volume also increases, and vice versa. Mathematically, Charles’s law can be expressed as:
V1/T 1= V 2/T 2
Where V 1and T 1represent the initial volume and temperature, and V 2and T 2represent the final volume and temperature, respectively.
Gay-Lussac’s Law
Gay-Lussac’s law states that the pressure of a gas is directly proportional to its absolute temperature at constant volume. This means that as the temperature of a gas increases, its pressure also increases, and vice versa. Mathematically, Gay-Lussac’s law can be expressed as:
P1/T 1= P 2/T 2
Where P 1and T 1represent the initial pressure and temperature, and P 2and T 2represent the final pressure and temperature, respectively.
Combined Gas Law
The combined gas law combines Boyle’s law, Charles’s law, and Gay-Lussac’s law into a single equation that relates all four variables (pressure, volume, temperature, and moles) for a gas. The combined gas law is:
P1V 1/T 1= P 2V 2/T 2
This equation can be used to solve a wide variety of gas law problems.
Applications of Gas Laws
Gas laws have numerous applications in chemistry and other fields. Some examples include:
- Calculating the volume of a gas at a different temperature or pressure.
- Predicting the pressure of a gas in a closed container when its volume or temperature changes.
- Determining the molar mass of a gas by measuring its volume and pressure under known conditions.
Phase Diagrams
A phase diagram is a graphical representation of the conditions under which a substance exists in different phases (solid, liquid, or gas). Phase diagrams show the pressure and temperature conditions at which phase transitions occur. They are useful for understanding the behavior of substances under different conditions and for predicting the phase of a substance at a given temperature and pressure.
FAQ Section
What is the ideal gas law equation?
PV = nRT
How can I use the kinetic molecular theory to explain the behavior of gases?
The kinetic molecular theory postulates that gas particles are in constant random motion, colliding with each other and the walls of their container.
What is gas stoichiometry?
Gas stoichiometry involves using the mole concept to calculate the mass, volume, or moles of gases involved in a chemical reaction.